Steel Protection
By Hot Dip Galvanizing & Duplex Systems |
|
Information courtesy of: HOT DIP GALVANIZERS
ASSOCIATION SOUTHERN AFRICA
Corrosion Resistance of Hot Dip Galvanized Coatings
The life of a hot dip galvanized coating is more or less proportional to its
thickness in a given environment. (Table 2).
Hot dip galvanized coatings on steel protect against corrosion in two Ways:
-
Barrier protection is provided by a virtually non-porous film which isolates
the steel substrate from corrosion inducing substances in the surrounding
environment.
-
Cathodic or sacrificial protection is provided at small uncoated surfaces
while corrosion creep under the surrounding coating cannot occur.
The corrosion rate of zinc is low in most environments. This is due to the
natural formation of a stable protective film of zinc conversion products which
develops on the surface of the coating.
12.1 CORROSION RESISTANCE IN THE ATMOSPHERE
When a hot dip galvanized article is withdrawn from the molten zinc, the coating
surface immediately reacts with oxygen and moisture to form combinations of both
zinc oxide and zinc hydroxide. Carbon dioxide in the atmosphere rapidly converts
these surface conversion products into a stable, tightly adhering, basic zinc
carbonate film with very low solubility. This ensures that farther attack of the
underlying zinc is prevented. The initial shiny surface with a metallic lustre
disappears to be replaced by a matt, light grey appearance (figure 74).
|

Figure 74. Exposed surface ala hat dip galvanized coating with outer layer of
pure zinc. The shiny surface disappears to be replaced by grey corrosion products
(sometimes called zinc patina).
|

Figure 75. Map showing atmospheric corrosion rate of mild steel and pure zinc
coatings in South Africa. CSIR Data. Areas C1 to C5 are more or less in terms of
SABS ISO 14713.
|
The atmosphere contains greater or lesser corrosive substances such as
chlorides. In marine environments and sulphur dioxide associated with industrial pollution. Humidity levels, rain patterns and condensation all
influence the degree of corrosion. The different factors can occur in favourable
or unfavourable sequences, one after another, alternately. or in combination
with each other.
It is normal to differentiate between
corrosion rates in:
-
rural environments
-
marine (coastal) environments
-
urban environments
-
industrial environments
(See figure 75 for atmospheric corrosion of zinc).
The atmosphere in cities and industrial areas contains various pollutants. These
are able to attack the stable zinc carbonate film producing more soluble products which can be washed away. Consequently the corrosion rate of galvanized
steel will accelerate. Modern environmental controls are resulting in lower
pollution levels and hot dip galvanizing offers good protection in locations
where previously limited coating life was experienced.

Figure 76. Discoloured surface on lighting column. Coating consists mainly of an iron/zinc alloy that grows out to the surface.
Iron is freed during corrosion,
which leads to rust formation.
It is only surface rust and is of aesthetic significance only. The bracket for
the traffic sign has a coating of pure zinc as an outer layer.
In marine environments the corrosion of zinc is influenced by the salt content
of the air. However, marine air contains small quantities of magnesium salts,
with good passivating influences. Corrosion is therefore not as great as may be
expected. The salt content of the air usually reduces quickly away from the
coast i.e. by 80% over the first 800m from the high water mark.
The colour of corrosion products varies according to the environment in which
they are formed. Marine environments give somewhat whiter corrosion products
compared with rural and urban environments. Corrosion products are usually
darkest in urban environments.
The corrosion of zinc is influenced by many factors. This means that a generally
applicable formula for corrosion rates can not be given.
The ubiquitous nature of hot dip galvanizing means that there is always a
product such as a lamp post or fence near a proposed future site that can be
used to predict future performance.
The Hot Dip Galvanizers Association have frequently been involved in the
assessment of the corrosive conditions prevailing at a particular site, prior to
the selection of the final coating specifications. Knowledge about the corrosion
of zinc, and corrosion rates in different environments, is therefore extensive.
Reddish-Brown Discolouration
Some hot dip galvanized steel can adopt a reddish-brown colour after a period of
exposure. After prolonged exposure, particularly in sulphur-rich atmospheres,
this discolouration can gradually turn black. The discolouration occurs mainly
on coatings of iron/zinc alloy on silicon-killed steels.
The source of discolouration is the corrosion of Fe/Zn alloy to form rust
together with humid air or rain water. Rust has a great ability to colour, and
even small amounts can cause considerable discolouration.
Sometimes when discolouration is severe it is easy to conclude that rust
protection has been greatly reduced, or completely destroyed. However, this is
seldom the case. The iron/zinc alloys give better protection (in some
environments up to 30-40%) to the underlying steel than pure zinc.
If appearance is important, discoloured surfaces can be painted (figures 30, 31
and 76).
12.2 WET STORAGE STAIN
Sometimes a white, floury and voluminous coating called wet storage stain, or
white rust, appears on galvanized surfaces (figure 77).
The coating forms on materials with newly galvanized, shiny surfaces and
especially in crevices between closely packed sheets, angles and similar
products. A pre condition is that the material is exposed to condensation or rain
water in conditions where the moisture cannot evaporate quickly. Zinc surfaces
that have already received a normal protective layer of corrosion products are
seldomly attacked.
When zinc coatings are exposed to air, zinc oxide and zinc hydroxide are formed.
Under the influence of carbon dioxide in the air these are converted to basic
zinc carbonates. If air access to the zinc surface is restricted, as in narrow
crevices, then the area receives insufficient carbon dioxide to enable the
normal layer of carbonates to form.
The wet storage stain layer is voluminous and porous, and attached only loosely
to the zinc surface. As a result, protection against continued attack does not
exist, Corrosion can therefore continue as long as moisture remains on the
surfaces. When wet storage stain has occurred the object should be stacked to
enable the surfaces to dry quickly. This will stop the attack and, with free
access to air, the normal protective layer will be formed. The wet storage stain
is gradually washed away and the coating acquires an appearance that is normal
for exposed, hot dip galvanized steel.
Since the product of wet storage stain is very bulky (about 500 times that of
the zinc from which it was formed), an attack can appear to be serious. However,
an attack of wet storage stain often has little or no significance on the
service life of the corrosion protection. In the case of very thin coatings
however, e.g. on electroplated objects, a severe attack of wet storage stain can
be of significance.
Wet storage stain is best avoided by preventing newly galvanized surfaces from
coming into contact with rain or condensate during transportation. Materials stored outdoors should be stacked so
that water can run off easily, so that all surfaces are well ventilated (figure
78). Temporary protection against wet-storage stain is obtained through
chromating or phosphating. Painting after galvanizing gives very good
protection.
|

Figure 77. Wet storage stain that has farmed between tightly packed angles.
|
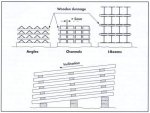
Figure 78. In order to avoid the formation of wet storage stain on newly
galvanized surf aces, profiled steel, beams and structures should be packed at
an angle and turned to prevent the
accumulation of water. Spacers are placed so as to avoid narrow crevices between
the zinc surfaces.
|
|

Figure 79. Galvanized bolt in contact with 3CR1 2 plate after 10 cycle SO2 test.
Note the cathodic protection provided by the galvanized bolt head to the
surrounding steel.
|
Wet storage stain which has already formed can be removed completely or
partially by moderate mechanical or chemical treatment. See "Removal of Wet
Storage Stain" page 17.
12.3 GALVANIC CORROSION
If two different metals or alloys, completely or partially surrounded by an
electrolyte, are connected, a galvanic cell is created, Which metal becomes
the anode or cathode is determined by their electrode potentials in the
electrolyte in question.
In sea water, which corresponds to the majority of practical conditions, some
metals and alloys take up different positions on the electrochemical scale,
shown in table 24.
If steel is connected to copper or brass the steel becomes the anode in the cell
and corrodes. However, if steel is connected to cadmium, aluminium, zinc or
magnesium, it becomes the cathode and is protected against corrosion, while the
anode metal is consumed (figure 79).
Galvanic corrosion is also called
bimetallic corrosion and is used to protect underwater structures from
corrosion, where it is termed cathodic protection.

Table 24. Electrochemical potential scale in sea water at +25C.
For maximum corrosion resistance under conditions of extreme humidity,
overlapping galvanized surfaces should be insulated from each other by the
application of an inhibitive jointing compound in accordance with SABS 305.
Alternatively a suitable paint may be used. Galvanized surfaces in contact with
other materials may also require insulation.
Galvanized members in contact with aluminium conductors may require the use of
an electrical conducting compound at joint faces, to repel moisture and inhibit corrosion.
|
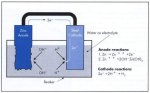
Figure 80. Galvanic corrosion a! zinc in contact with steel in water.
|

Figure 81. After 20 years of marine exposure, this site cut unrepaired hot dip
galvanized steel grating still offers cathodic protection at the cut ends.
|
|
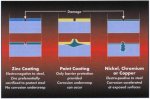
Figure 82. Schematic diagram to illustrate the consequences of damage to
different types of coatings
offering corrosion protection.
|
Cathodic Protection Afforded by Zinc Coatings
In hot dip galvanized steel, zinc and steel are in good electrical contact with
each other. If the zinc coating is damaged in the presence of an electrolyte a
galvanic cell is created, The electrolyte could be condensate or rain water.
Sometimes the entire structure can be submerged in liquid. In this cell the zinc
becomes the anode i.e. corrodes, the exposed steel becomes the cathode and is
therefore protected from corrosion (figure 80).
In the initial phase it is possible to see a weak rust formation on the exposed
part of the steel surface where the coating has been damaged, but after a while
whitish-grey areas form which gradually spread over the entire damaged area
(figure 81). The zinc coating corrodes and sparingly soluble zinc alloys descend
to the cathode surface where they protect the steel from continued rust attack.
This is often called self-healing, which is something of a misnomer since the
zinc layer is, of course, not restored.
In case of exposure in water the zinc salts do not always precipitate at the
point of damage since they can be flushed away by movement in the water. The
protective action remains, however, provided that the steel surface is not too
large. The steel is protected by the electrical current generated in the galvanic cell when the zinc corrodes.

Figure 83. Stainless steel fasteners attached to hat dip galvanized plate in
immersed conditions,
note the sacrificial attack of the zinc coating
surrounding uninsulated fasteners compared with the insulated fastener where no
attack of
the surrounding zinc has taken place.
Owing to the cathodic protection generated by the zinc, rust cannot creep in
under the coating at the point of damage in the way that it can creep under
films of paint or coatings of metals more noble than steel (figure 82).
Zinc coatings on steel are unusual, since a fairly large area of damage to the
coating does not cause catastrophic corrosion. The range of cathodic protection
is dependent on the nature of the electrolyte that creates the cell. For
structures in normal atmospheres it is usual to expect protective action over
several millimeters. However, in sea water significantly greater distances can
be expected.
Zinc Coatings in Contact with Non-Ferrous Metals
Aluminium and stainless steel can often be connected directly to galvanized
material in air or fairly dry environments without noticeable corrosion taking
place. However, in water an insulator should always be used to prevent
accelerated corrosion of the zinc (figure 83).

Figure 84. Brass bolt in hot dip galvanized steel on a parking deck.
Copper and copper alloys are more electrically active, and there is often a
release of copper ions which spread over large surfaces and cause noticeable
attack. For this reason, these metals should never be allowed to come into
contact with galvanized steel, and an insulator should always be used (figure
84).
12.4 CORROSION RESISTANCE OF
HOT DIP GALVANIZED
COATINGS IN AQUEOUS
CONDITIONS
General
Zinc carbonate, the protective film formed over a hot dip galvanized coating, is
relatively insoluble in water. However, this stability is restricted to an
acid/alkali pH range of 6 to 12.5. Zinc is amphoteric in nature; that is, it
forms soluble salts at low and high pH values. This is clearly shown in figure
85.
Notwithstanding the above, water contains numerous dissolved salts as well as
carbon dioxide and oxygen in solution. Organic matter can be picked up by water
as it passes over vegetation. This can also be a major contributor to corrosion in some instances. The effects of water quality on the corrosion rate are
summarized in figure 86.
In soft waters, zinc corrosion is accelerated. Also, the tolerance for chloride
salts is reduced. A reserve alkalinity level is required to stabilize the zinc
carbonate film, This is generally assumed to be of the order of 50 - 75mg/l (as
CaCO3). In hard waters, high chloride levels (> 2000mg/l) can be tolerated.
Sulphates, nitrates and phosphates are generally considered to be protective
towards hot dip galvanizing. However,
when combined with ammonia compounds (such as with fertilizers) soluble zinc
compounds may be formed and acid conditions can arise causing attack of hot dip
galvanized steel. Organic compounds such as tannins will arrest the corrosion of
hot dip galvanized steel but the settling of solids can create conditions for
crevice corrosion. Similarly, slime build-up should be avoided as microbially
induced corrosion (MIC) can occur, leading to rapid attack.
Flow rates should be maintained at sufficiently high levels to ensure that all
debris is held in suspension rather than allowed to settle. It should be
considered "good practice" to flush systems on a regular basis. This
should be carried out on all fire protection systems although, as the water
entering these systems is generally of good quality, corrosion rates tend to be
low provided that MIC does not occur in all instances, the corrosion performance
of galvanized piping in fire protection systems is far superior to that of bare
steel. Crevice corrosion is likely to occur where sediment becomes dense and
compacted. This may result in the provision of anaerobic sites suitable for the
start of MIC.
Under normal circumstances the amount of dissolved oxygen in a water would be
sufficient to ensure that no deleterious effects occur, However, anaerobic or
septic conditions can affect hot dip galvanized piping adversely as is the case
with other metals. For drinking water purposes some form of chlorination is
generally applied. Therefore, in normal distribution systems anaerobic
conditions giving rise to MIC, should not occur. It is important when testing water lines that clean water be
used and the system drained if it is to be left unused for some time.
Chlorination has no effect upon the protection properties of galvanizing. High
oxygen levels accelerate the corrosion rate of zinc. Similarly, high carbon
dioxide levels tend to produce add conditions, which can accelerate corrosion in
flowing systems.
|
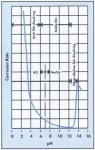
Figure 85. The influence of pH an the corrosion rate of zinc in aerated (CO2
free) solutions.
(Dilute HCl and NaOH at 30C). Note: The curve only applies far continuous
exposure under the specific conditions. Far other conditions it can be used as a
guide. In hard/scale forming waters protective layers are
farmed which greatly alter the curve.
|

Figure 86. Effects of water quality on the corrosion rate
of a hot dip galvanized
coating.
|
Effect of water temperature
Hot dip galvanized piping has been used for hot water supplies with no
deleterious effects in many applications. However, when used above 65C the zinc
is no longer protective to exposed steel. It is therefore recommended that hot
dip galvanized systems not be used above 65C.
The electricity supply commission (Eskom), advise that with proper pipe
insulation, the maximum temperature for hot water cylinders be 60C. For
practical purposes therefore, hot dip galvanized piping is acceptable for use in
both hot and cold water systems.
In domestic systems copper should only be used downstream of hot dip galvanized
piping. This will avoid the possibility of pitting corrosion.
|
No. |
Aggressiveness |
Soil
Condition |
Resistivity
in ohm |
Method of protection |
| 1 |
low |
dry |
> 100 |
Hot dip galvanizing> 200m |
| 2 |
low |
moist |
> 450 |
Hot dip galvanizing > 200m |
| 3 |
moderate |
dry |
< 100 |
Hot dip galvanizing> 200m plus
a rust allowance an the basis
material of 0.5mm on each side. |
| 4 |
moderate |
moist |
150 - 450 |
Same as for 3 |
| 5 |
high |
moist |
50 - 150 |
Hat dip galvanizing > 200m and
rust allowance of 1mm an each side. |
| 6 |
very high
|
moist
(In certain
cases
H2SO4
can form) |
< 50 - 100 |
Same as for 5 but rust allowance
of 1.5mm an each side |
Table 25. Sail aggressiveness at different resistivity levels with hat dip
galvanized coatings.
Effect of sea water
Hot dip galvanized coatings perform relatively well in submerged seawater
conditions which are severely corrosive to most protective systems. Dissolved
salts present in seawater react with zinc to form a protective layer minimizing
corrosive action. The pH of seawater tends to be constant worldwide as a result
of the buffering action of the hydrogen-carbonate salts present. The presence of
pollutants is equally not detrimental provided that levels are within
internationally acceptable norms.
A simple nomogram (table 27) has been produced to allow the specifier to
determine the suitability of hot dip galvanizing for the protection of steel
piping in water. This provides guidance based upon the water quality and general
operating conditions likely to be encountered. More detailed information is
contained in SABS 0374-1: The suitability of hot dip galvanized steel piping for
the transportation of potable water.
12.5 CORROSION RESISTANCE OF
HOT DIP GALVANIZED
COATINGS IN SOIL
CONDITIONS
Soil can contain weathered products, free or bound salts, acids and alkalis,
mixtures of organic substances, oxidizing or reducing fungi, micro-organisms,
etc. Depending on its structure, soil has different degrees of permeability to
air and moisture. Normally, the oxygen content is less than in the air, while
the carbon dioxide content is higher. The corrosion conditions in soil are
therefore very complicated and variations can be great between different
locations, even those in close proximity to each other.
Southern African soils vary from highly corrosive in some regions to moderately corrosive
in others.
One method of determining the corrosivity of a soil is to measure its
resistivity. Recommendations are given in table 25.
If the resistivity of the soil cannot be determined, the rule-of-thumb method
listed in table 26 can give a measure of guidance. Where the exposure of metals
to soil is concerned, it is advisable to seek expert advice from suitably
qualified sources.
See also "Guidelines for Burled Hot Dip Galvanized Conveyance Piping" -
available from the Association.
12.6 HOT DIP GALVANIZED STEEL IN CONTACT WITH BUILDING
MATERIALS
Mortar, Plaster and Wood
Damp mortar and plaster attack zinc. The attack ceases when the material dries
out. Dry or moderately damp wood, both impregnated and unimpregnated, can be
nailed with hot dip galvanized nails to good effect, However, in the case of
nails or threaded unions that are constantly exposed to water an acid-resistant
material is preferred. Other dry building materials, such as mineral wool, do
not attack zinc.
Wood with acidic properties should not come into contact with galvanized steel.
Concrete
Unprotected reinforcement can corrode in certain environments when moisture
penetrates the concrete through cracks and pores. Since rust has a greater
volume than the steel from which it was formed, the covering layer over the
reinforcement can crack and spall (figure 88).
Steel components such as bolts and
edge guards that have been partly grouted in are often poorly protected against
rust. Apart from crack formation and scaling, a problem occurs with unsightly
rust staining on the concrete surfaces below.
|
Soil type |
Aggressiveness |
| Lime, calcareous marl,
moraine, sand marl |
Low |
|
Sand, gravel |
Moderate |
| Clay, peat bag,
humus-rich soils |
High |
Table 26. Corrosivity of different soil types.
| VALUE |
PARAMETER |
UNIT |
RATING |
| CONDITION OF WATER |
A
|
Flowing
Standing
Anaerobic |
|
2
1
-5 |
| CORROSIVITY
INDEX * |
B
|
< 1
≥ 1, < 2
≥ 2, < 5
≥ 5 |
|
0
-1
-2
-4 |
|
TOTAL ALKALINITY |
C
|
< 50
≥ 50, < 200
≥ 200, ≤ 300
> 300 |
ppm as (CaCo3)
|
-1
1
0
-1 |
|
CALCIUM HARDNESS |
D
|
< 50
≥ 50, < 200
≥ 200 |
ppm as (CaCo3)
|
-1
2
3 |
| pH |
E
|
< 5.5
≥ 5.5, < 6.5
≥ 6.5, ≤ 7
> 7 |
|
-6
-4
-1
1 |
|
CALCIUM CARBONATE PRECIPITATION INDEX |
F
|
< -2
≥ -2, < 0
0
> 0, ≤ 6
> 6 |
|
-2
-1
0
1
0 |
| Probability = Sum
(A to F) |
Result
Greater than 1
1 to -1
-3 to -5 |
Performance
Satisfactory (+25 years)
Fair
Unsatisfactory |
* Corrosivity index (B) can be
calculated by -
(C1 x 0,03) + (SO4 x 0,04) |
Table 27. Probability of performance.
This kind of damage can be avoided if the reinforcing steel is hot dip
galvanized (figure 87). Hot dip galvanized reinforcing steel or mesh can
therefore be used in grouted facade sections. One of the advantages of this is
that there is no risk of rust runs discolouring the facade.

Figure 87. Flat dip galvanized reinforcing bars prior to casting concrete,
marine conditions.
According to the Building Research Establishment in the UK, the average adhesion
for smooth reinforcement steel in concrete is as follows:
|
hot dip galvanized steel |
3.3-3.6 MPa |
|
black steel |
1.3-4.8 MPa |
The large range for black steel stems from different degrees of rust and
compositions of oxide scale.
According to work done in Finland, the stress for 0.1 mm of slip in
reinforcement bars in concrete is approximately as follows:
|
black steel |
150 MPa |
|
hot dip galvanized steel |
160 MPa |
| hot dip galvanized and
chromated steel |
190 MPa |
When concrete is cast its pH value is around 13. At this high pH, fresh zinc is
attacked and hydrogen is produced, which could give rise to poor adhesion.
However, the attack ceases as soon as the concrete has hardened and any residual
pores are not harmful.
In order to avoid fresh zinc surfaces coming into direct contact with wet
concrete it is advisable to allow the galvanized steel to age for several weeks.
The cover layer of basic carbonates which then appears will minimize both attack
and the production of gas, and will also promote adhesion. Another common way of
preventing attack from fresh concrete is to chromate the galvanized steel. A
further alternative is to add about 40 ppm (by mass) of chromates, to the water
when concrete is mixed.
12.7 ABRASION RESISTANCE OF HOT
DIP GALVANIZED COATINGS
Pure zinc is a soft metal, even though it is harder than most organic coating
materials. The iron/zinc alloys produced in hot dip galvanized coatings are,
however, very hard. In fact, they are harder than ordinary structural steel
(figure 89).
The alloys are therefore more resistant to abrasion than pure zinc and
experiments have shown that the alloy layer has a resistance to abrasion 4-5
times that of pure zinc.
Hot dip galvanized articles are often used when the surface is to be subjected
to abrasion. Examples of this include stairs, floor hatches, hand railings, grid
flooring and walkways (figure 5).
12.8 HOT DIP GALVANIZED
COATINGS EXPOSED TO
ELEVATED TEMPERATURES
Conventional zinc coatings can be exposed continuously to temperatures up to
about 200C and non-continuously to temperatures of up to 350C.
At sustained temperatures in excess of 200C a diffusion reaction begins inside
the coating and causes the outer layer to split-off from the underlying
iron/zinc layer. However, the iron/zinc layer has a very good resistance to
corrosion and can, depending on its thickness, protect the steel from rust for a
very long time.
Aluminium-alloyed zinc layers on thin sheet can resist even higher temperatures.
Aluzinc and galvalume for instance, can withstand sustained temperatures up to 315C.
|

Figure 88. Spoiling of the concrete layer an reinforcing steel in a concrete
bridge balustrade.
|

Figure 89. Microsection of a hat dip galvanized coating showing variations in
hardness through the coating.
|


















